Research Group Martin
The RG Martin is interested not only in developing individualized cell and gene therapies for cardiac and pulmonary diseases, but also in investigating disease mechanisms for development of new pharmacologically active ingredients, and finding alternative methods to animal experiments. Therefore, the following research areas are addressed in the RG Martin:
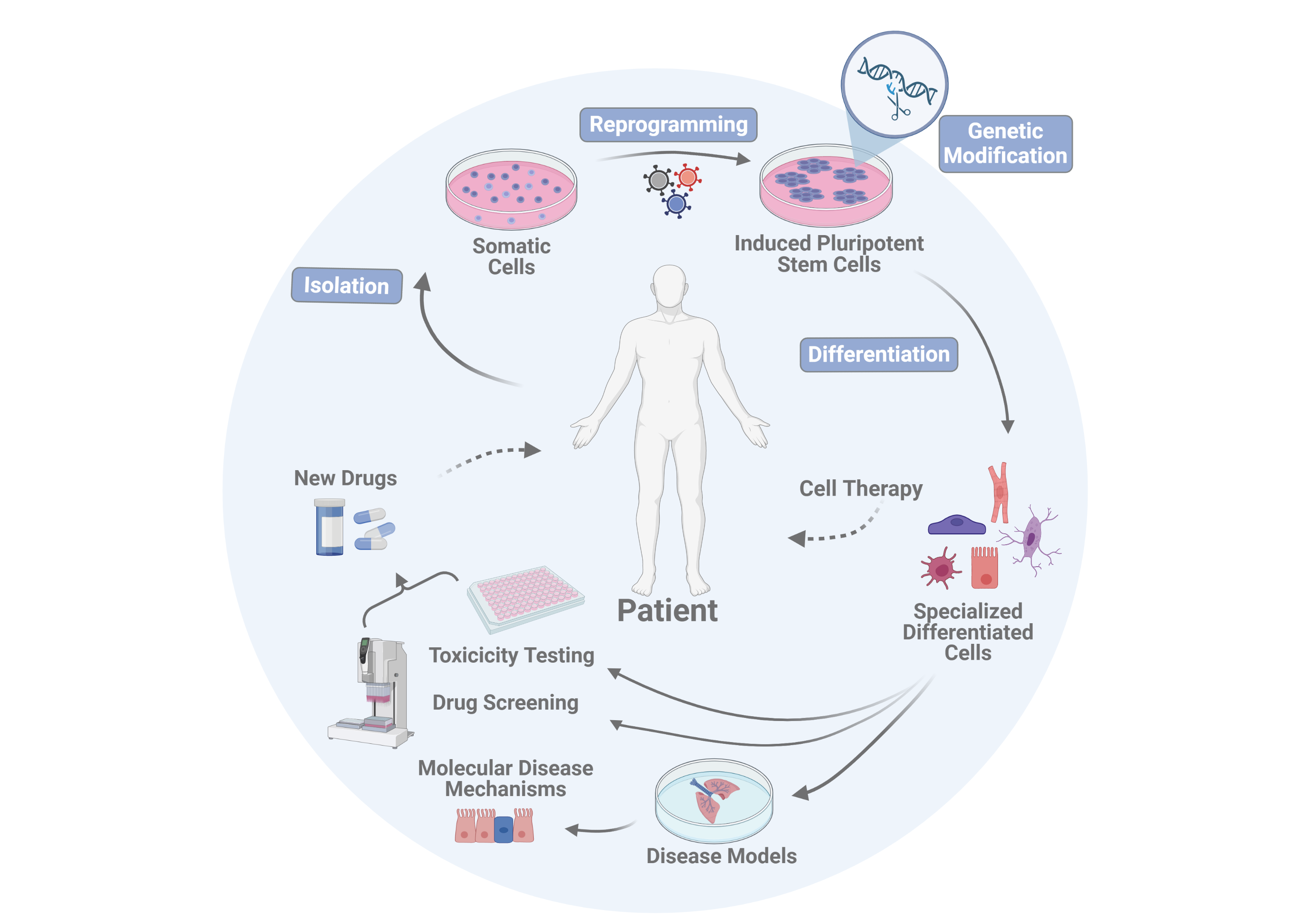
1. Reprogramming of somatic cells into clinically applicable induced pluripotent stem cells (iPSCs)
2. Generation of patient or disease-specific iPS cell lines
3. Genetic modification of iPS cells and iPS cell-derived cell types
4. Optimization of vascular and respiratory differentiation processes of iPS cells for the development of organotypic in vitro disease models
5. Investigation into the safety of cell therapies
6. Development of methods for in vivo correction of disease-specific mutations via "gene editing"
1. Reprogramming of somatic cells into clinically applicable induced pluripotent stem cells (iPSCs)
In the research group of Prof. Martin, the reprogramming of somatic cells derived from different species is well-established but is also continuously being improved.
The first step of reprogramming somatic cells into iPS cells is the introduction of a defined combination of different cellular factors (reprogramming factors), usually with the help of so-called viral vectors. The introduction of reprogramming factors into differentiated cells triggers a cascade of genetic events, including alteration of the DNA methylation status, chromatin remodeling, and modification of gene expression, finally resulting in the conversion of the fully differentiated somatic cell into a pluripotent state.
One goal of the RG Martin is the development of clinically applicable protocols for the efficient generation of iPS cells. To guarantee the safety of potential future clinical applications of iPSC-based regenerative cell therapies, we improve our manufacturing protocols for GMP-compatible iPS cell products continuously.
As an alternative, transgene-free reprogramming methods, such as synthetic mRNAs or non-integrating RNA viruses (e.g. Sendai virus), are used to avoid the activation of proto-oncogenes and the formation of teratomas (germ cell tumors). To address an additional safety concern of the therapeutic use of iPS cells and their derivatives, we are establishing our own improved Sendai viral vector system in the Martin group. This system can be used for the delivery of various factors, such as reprogramming factors into the target cells, as well as for targeted genetic modification of therapeutically applicable cells (see also “6. Development of methods for in vivo correction of disease-specific mutations via "gene editing")
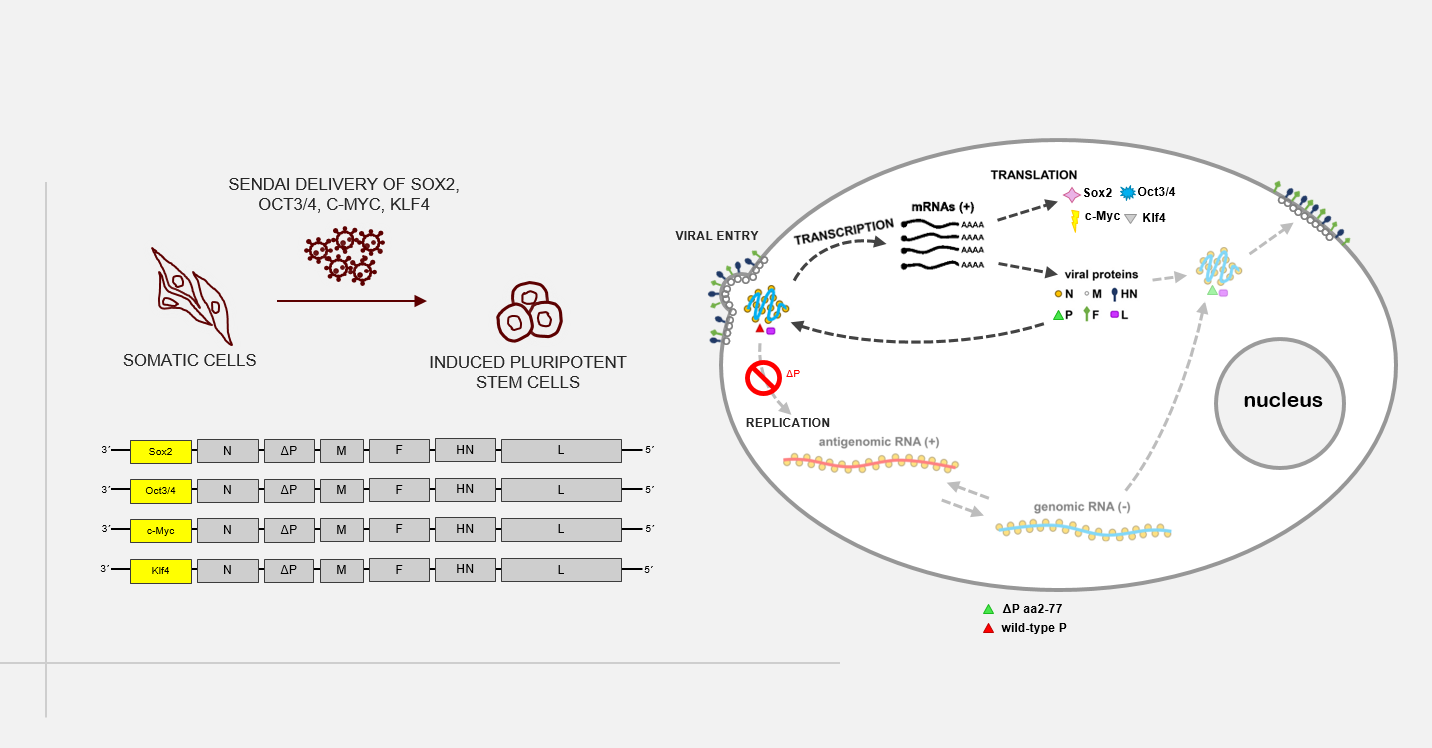
A novel, replication-deficient Sendai virus vector system for the generation of induced pluripotent stem cells
Commercially available reprogramming vectors based on Sendai RNA virus (SeV), a paramyxovirus without a DNA-state, are widely used to generate induced pluripotent stem cells (iPSCs). Unlike DNA vectors, SeV cannot integrate into the genome of infected cells; paramyxoviruses replicate and transcribe exclusively in the cytoplasm. Furthermore, recombination events have never been observed among paramyxoviruses. Available SeV reprogramming vectors, however, are replication-competent and prolonged expression of reprogramming factors requires extended culture periods to completely eliminate virus RNA entirely. In this study, we aim at developing a new reprogramming system based on a formerly described replication deficient SeV vector (rdSeV; Bossow, 2012, OpenVirolJ). Based on this vector system, we have generated four reprogramming vectors (rdSeV-Sox2, rdSeV-Oct4, rdSeV-c-Myc, rdSeV-Klf4) and were able to achieve high production titers (up to 10^8 IFU/mL). Notably, qRT-PCR analysis of transduced CD34+ cells showed high transgene expression despite the fact that secondary transcription is disabled in our vector system. Expression was detectable as early as 12 hours post-transduction and persisted for at least 7 days. We are currently optimizing vector ratios and individual vector titers to achieve efficient reprogramming of somatic cells. In the long run, this system could be a safer alternative for the generation of clinically applicable iPSCs. On the practical side, it might facilitate reprogramming since no extended culture period is required and viral RNA is diluted earlier than in available systems.
2. Generation of patient or disease-specific iPS cell lines
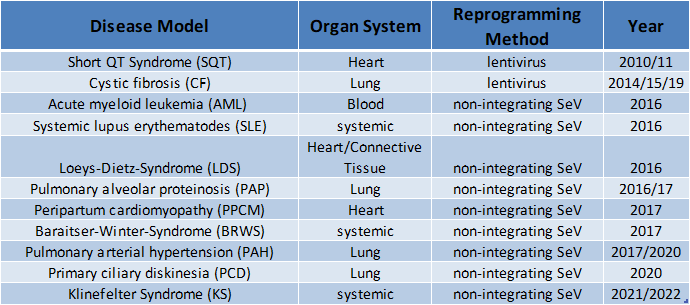
In the Martin group, iPS cells have so far been produced from cells from mice and humans, but also from various primate species (macaque, gorilla, bonobo). Various cell types produced from these cells, such as blood cells, endothelial cells, epithelial cells, etc., have been or are used in our working group or by cooperation partners to deal with very different questions, such as the investigation of brain development.
Meanwhile, a large number of human patient- or disease-specific iPS cell lines have been established in the research group. This collection of iPS cell lines is constantly being expanded and is now available for various research projects and in particular for investigations of the pathomechanisms of various diseases.
3. Genetic modification of iPS cells and iPS cell-derived cell types
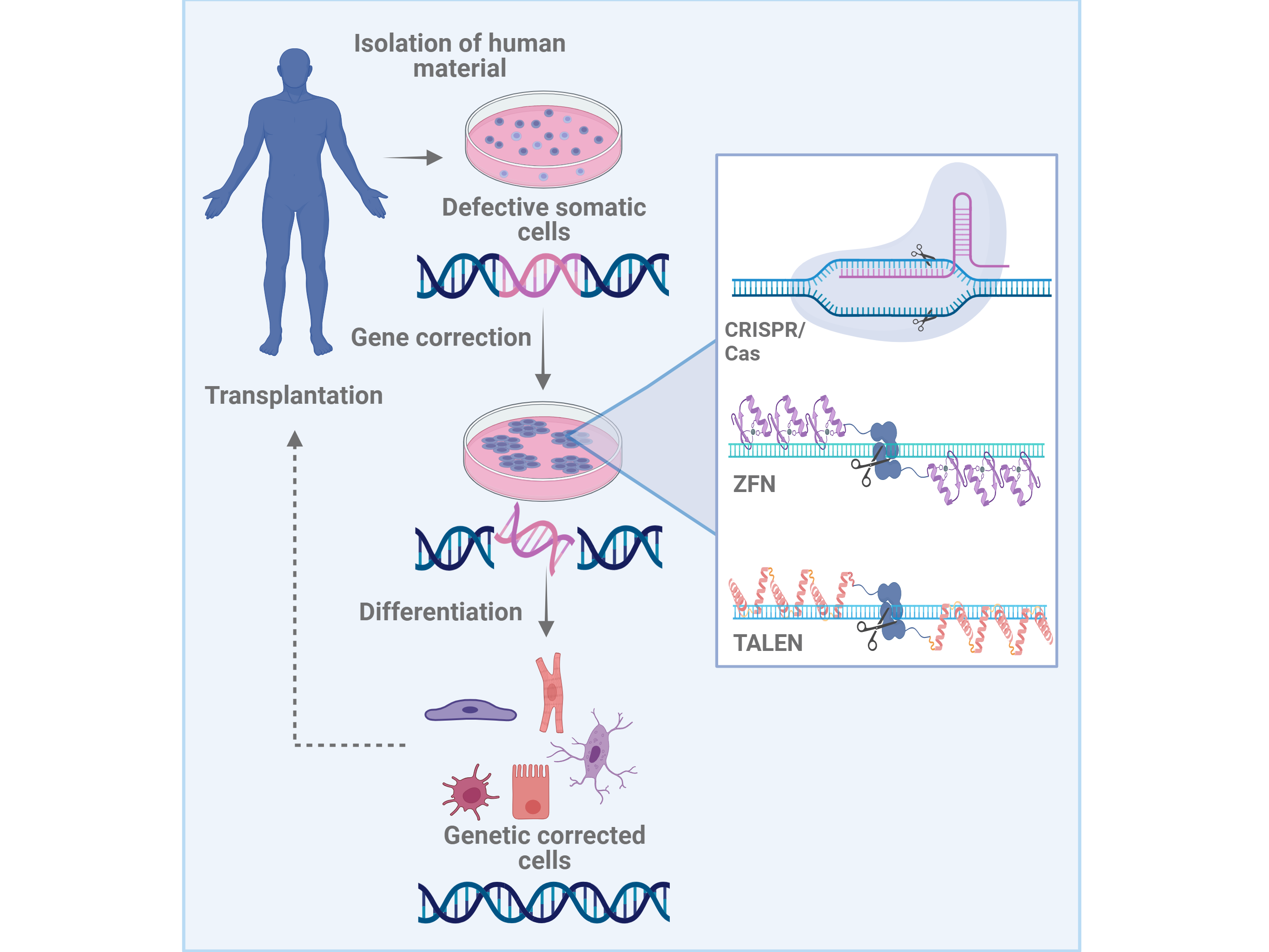
Many cardiovascular diseases, e.g. of the heart or the respiratory tract, are associated with genetic defects or mutations. In principle, regenerative therapies are conceivable, in which patient-specific cells are obtained, reprogrammed into iPS cells, and then genetically corrected. So far, genetic modifications of stem cells have primarily been performed using randomly integrating viral vectors or plasmids. However, these methods risk the activation of neighboring alleles, including proto-oncogenes, via insertional mutagenesis. Thus, for future clinical applications of patient-specific iPS cells, alternative gene modification methods that are associated with lower risks must be used.
Novel technologies for targeted genome modification are based on the use of artificial enzymes called "designer nucleases.” These enzymes include zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and the CRISPR/Cas9 system. These enzymes can be engineered to insert a DNA double/single-strand break in a specific locus, allowing for the targeted modification of the genome and correction of genetic defects with the help of the cell's own repair mechanisms. Compared to conventional gene targeting and the use of integrating vectors, the ZFN, TALEN or CRISPR/Cas9-based homologous recombination is not only significantly safer, but also much more efficient. Together with the groundbreaking discovery of iPS cells, these enzymes could make it possible to develop patient-specific cell therapies for many diseases, particularly those which are genetic.
Furthermore, in combination with the ZFN, TALEN or CRISPR/Cas9 methods, gene constructs containing therapeutic genes can be introduced into the cells to produce "suprafunctional" cells. Reporter or selection genes under the control of appropriate cell-type-specific promoters can also be expressed. These systems can be of great benefit not only for optimizing the culture and differentiation of pluripotent stem cells, but also for the purification of cells differentiated from stem cells, e.g. cardiomyocytes and lung epithelium.
In addition, transgenic reporter cell lines can be used for the screening of new active substances for the treatment of various diseases. Designer nucleases are a valuable tool for reporter cell lines which will be used in high-throughput screening. They allow transgenic reporter constructs, that facilitates an automated screening, to be efficiently and specifically introduced into disease-specific iPS cells.
4. Optimization of vascular and respiratory differentiation processes of iPS cells for the development of organotypic in vitro disease models
The in vitro differentiation and production of iPS-derived lung and blood vessel cells must be highly optimized for their use in organotypic disease models and screening systems, as well as in cell-based regenerative therapies.
Even though extensive information about developmental biology is already accessible, the regulation of differentiation processes in complex organs such as the lungs is not yet sufficiently understood. However, for a targeted differentiation via sequential inhibition or activation of the signaling pathways involved, a deeper understanding of these processes is absolutely necessary. Modern high-throughput sequencing methods, in particular single-cell RNA sequencing, which can be used to identify activated signaling pathways and misguided subpopulations, also support this need.
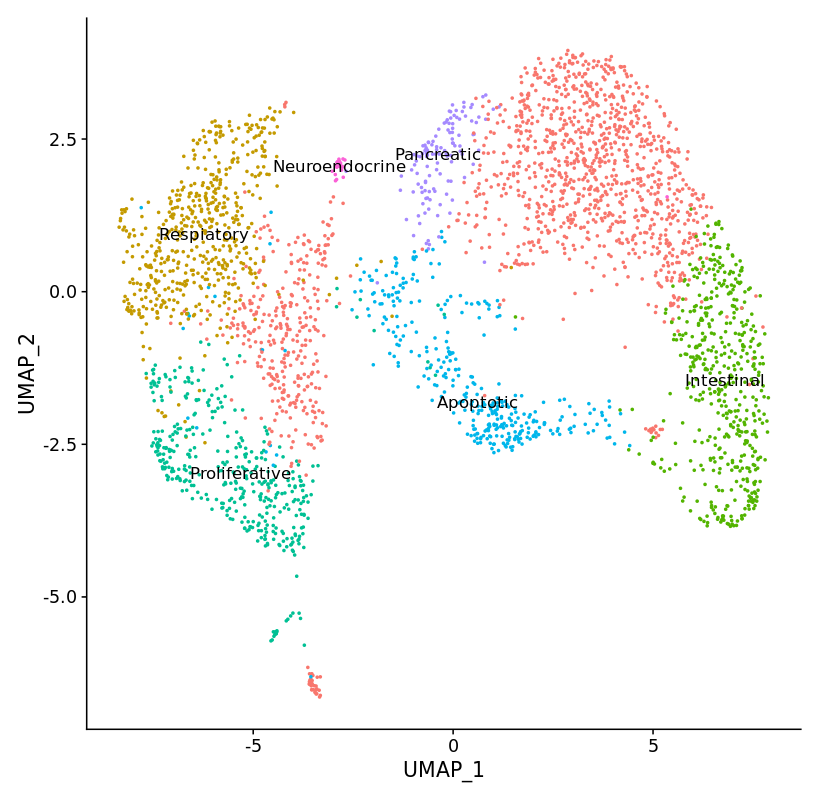
Application of single cell RNA sequencing to characterize transient cell populations and molecular signalling during respiratory differentiation of human pluripotent stem cells
Complex protocols have been developed to differentiate human induced pluripotent stem cells (hiPSC) via endoderm, foregut and NKX2.1pos early respiratory progenitor cells towards mature respiratory epithelia. However, these protocols reflect human development to a limited extent, efficiency and robustness of the protocols still need further improvement, and protocols have to be adapted for each individual cell line. At this point, it is not clear to what extent cell cycle characteristics and epigenetic features of individual lines play a role, and how paracrine factors released by contaminating cell lineages influence the differentiation outcome.
Therefore, further exploration of cellular composition and molecular signaling of the cells differentiated according to the established protocol are necessary to reveal contaminating cell types and potential signaling pathways to be targeted for the further improvement of the differentiation protocol.
hiPSCs were differentiated towards early respiratory progenitors according to the established protocol. Single cell RNA sequencing data analyses have been initiated from different stages of the differentiation protocol. Data obtained on day 12 of differentiation revealed not only lung progenitors (NKX2.1pos) as one major cell population, but in addition a second major discrete cell population identified as non-lung cells expressing CDX2 – a posterior endodermal and especially intestinal marker. Accordingly, differentiation cultures at endoderm / foregut endoderm stage have to be further manipulated to prevent formation of posterior foregut and midgut lineages.
Further analysis of different stages of the respiratory differentiation protocol is ongoing and resulting data will provide the ground for further protocol improvement via activation / inhibition of relevant signaling pathways
_________________________________________________________________________________________________________
In further studies, we are investigating why different cell types produced from iPS cells, which are a "juvenile" cell source, unexpectedly only have a comparatively low proliferative potential. We are currently investigating the phenomenon of early senescence in endothelial cells differentiated from iPS cells. Our goal is to identify the causes of premature senescence in iPS derivatives, to improve differentiation protocols and ultimately produce cell cultures with a higher proliferative capacity, for use in cell replacement therapies.
Investigation of mechanisms underlying premature senescence in human iPSC-derived endothelial cells
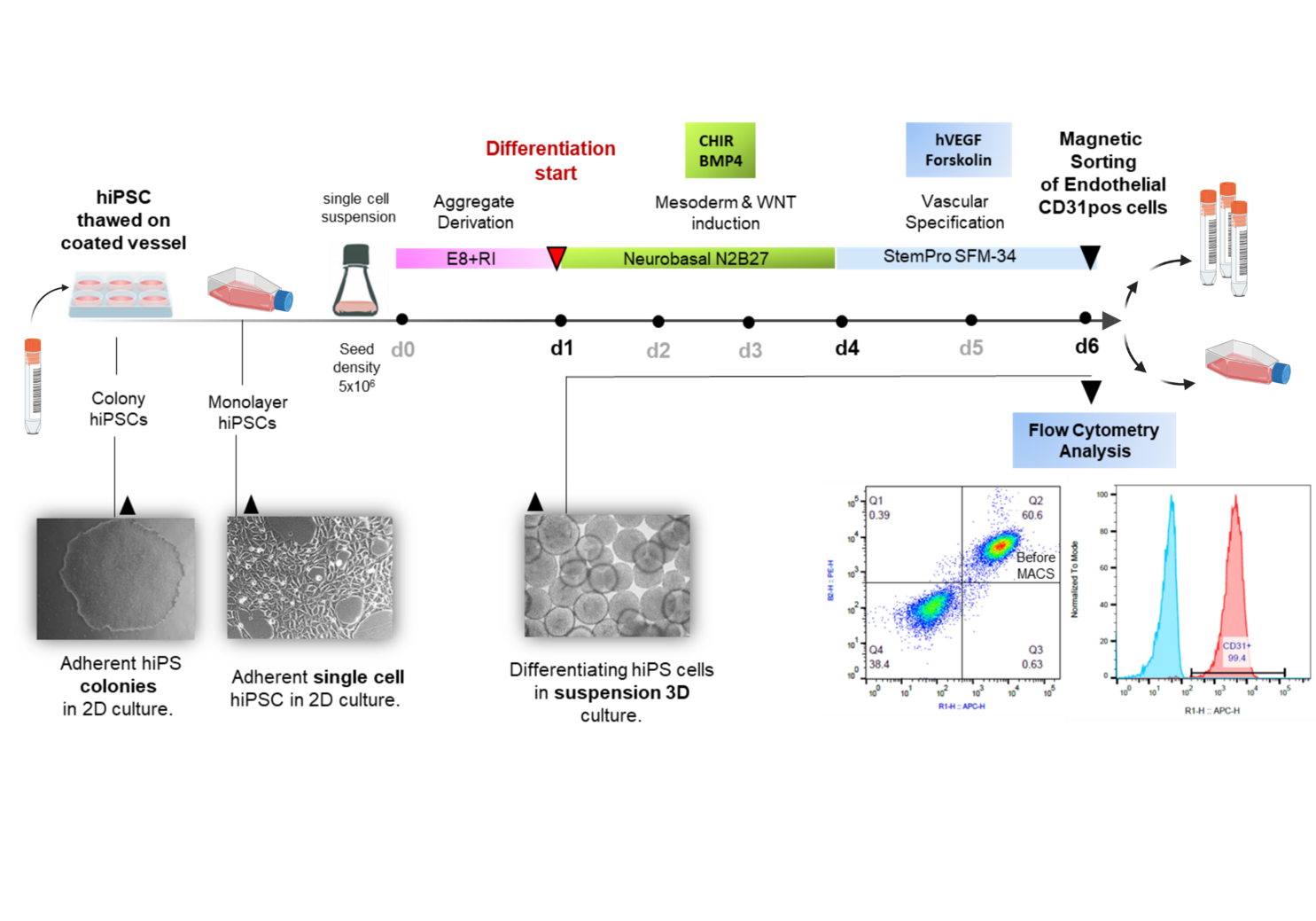
Induced pluripotent stem cells (iPSCs) are considered as potentially superior cell source for in vitro assays & cell therapies. Since protocols for differentiation and enrichment of specific cell lineages are typically still laborious and costly, the possibility to expand the resulting differentiated progeny would substantially facilitate the manufacturing process.
We and others have recently developed protocols for efficient production of highly enriched endothelial cells from hiPSCs (hiPS-ECs) that express common EC markers and display typical functionality. Contrary to their embryonic origin and to expectations, it is observed that hiPS-ECs differentiated from most lines show low population doublings with low expansion potential in comparison to primary human umbilical vein endothelial cells (HUVECs) or ECs obtained from adult individuals (HAOECs and HSVECs). The early senescence of iPSC derivatives has been observed in current protocols and the underlying reason has not yet been explained.
It is therefore the aim of our ongoing study to explore the underlying cause of the observed premature senescence in hiPSC-ECs for improving differentiation protocols and overcoming senescence for production of better proliferating ECs lacking senescence phenotype for use in cell therapy.
Our studies may represent the basis for improvement of current differentiation protocols yielding to differentiated hiPSC-derivatives with higher potential for culture expansion in future.
___________________________________________________________________________________________________________
Vascular and respiratory cell types produced from iPS cells are used in the Martin group both for disease modeling in in vitro systems and for screening of new active drugs in the therapy of various lung diseases.
We are involved in the development of therapies for the treatment of pulmonary hypertension and primary ciliopathies.

Our research also focuses on cystic fibrosis (CF). This disease is caused by mutations in the so-called “Cystic Fibrosis Transmembrane Conductance Regulator” (CFTR) gene.
As no effective treatment targeting CF is available to date, one of our research projects focus on the development of an innovative cell replacement therapy in which alternative ion channels are used.
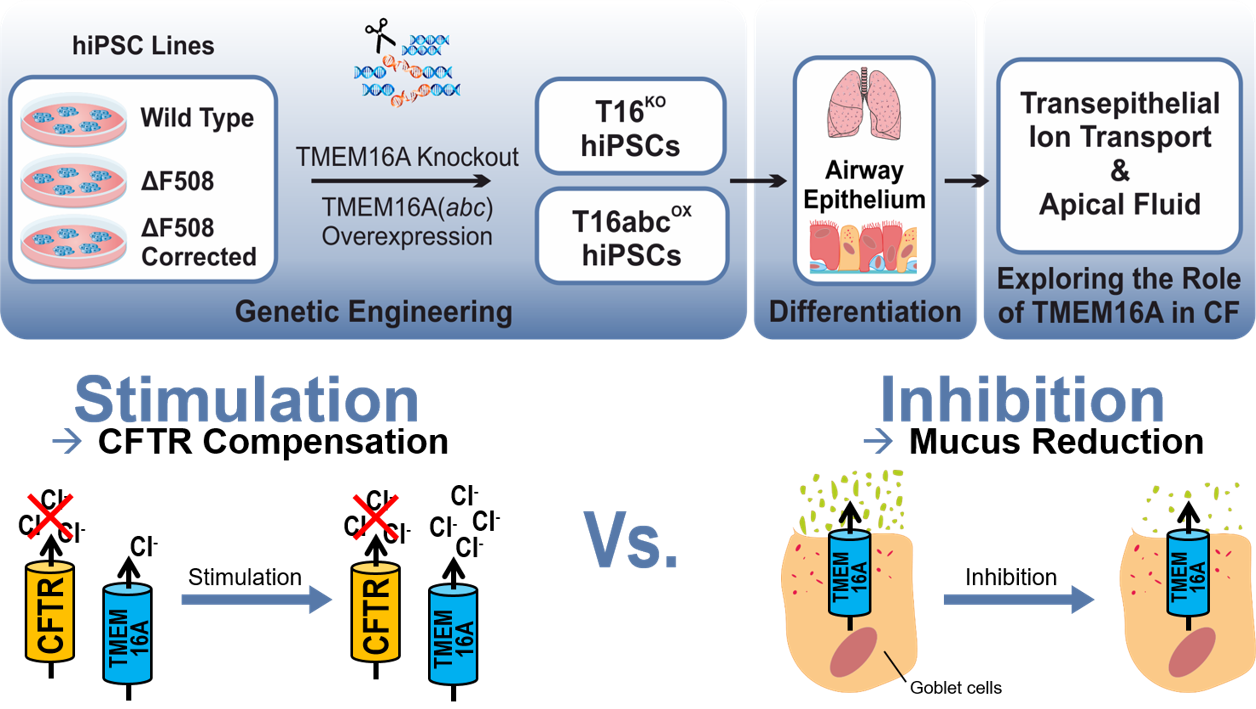
Evaluation of TMEM16A as a modifier for Cystic Fibrosis lung phenotype utilizing CF-patient specific human induced pluripotent stem cells
Cystic fibrosis (CF), also known as Mucoviscidosis, is a rare genetic disorder that causes the dysfunction of the chloride channel CFTR. In CF, the mutant CFTR leads to a disturbed chloride transport in the epithelial tissues of the human body and most severely affects the lungs. Here, the disturbed chloride secretion causes a dehydration of the airway mucus followed by chronic infection and inflammation that result in progressive loss of lung function. Although, CF therapy greatly advanced over the last decades, the effective therapy options for CF patients with rare CFTR mutation are mostly lacking. Previous studies have suggested that a co-expressed chloride channel known as TMEM16A may serve as a novel target in therapy of CF lung disease of all CF patients. However, it is still controversially debated whether the CF lung phenotype could be improved by stimulation of TMEM16A to induce mucus rehydration, or by inhibition of TMEM16A to reduce the mucus release. To resolve this issue, TMEM16A knockout (T16KO) as well as overexpressing (T16abcOX) hiPSCs were generated from multiple cell lines (wild type, CF patient-specific, gene corrected) and further differentiated into airway epithelial cells in air-liquid-interface (ALI) cultures. In close collaboration with the research group of Prof. Dr. Marcus Mall (Charité, Universitätsmedizin Berlin) functional studies are being conducted to measure the transepithelial ion conductance, ciliary beating and mucus properties in these transgenic hiPSC-derived airway epithelial cells. Our studies will create unique insights into the function of TMEM16A in CF and will hopefully provide conclusive findings about its utilisation in CF therapy. The project is funded by Mukoviszidose Institut GmbH
___________________________________________________________________________________________________________
IPS-based vascular and pulmonary cells, produced under optimized differentiation protocols can be used for future innovative cell replacement therapies. Patient-specific iPS cells have already been produced, characterized, and genetically corrected using designer nucleases, and the subsequent differentiation of the pluripotent cells into (precursor) cells of the airways has progressed greatly. However, effective replacement of the endogenous mutated stem cells of the respiratory tract are still in the early stages of development.
5. Investigation into the safety of cell therapies
Important development in the field of safety aspects of cell therapies is also carried out in the Martin research group.
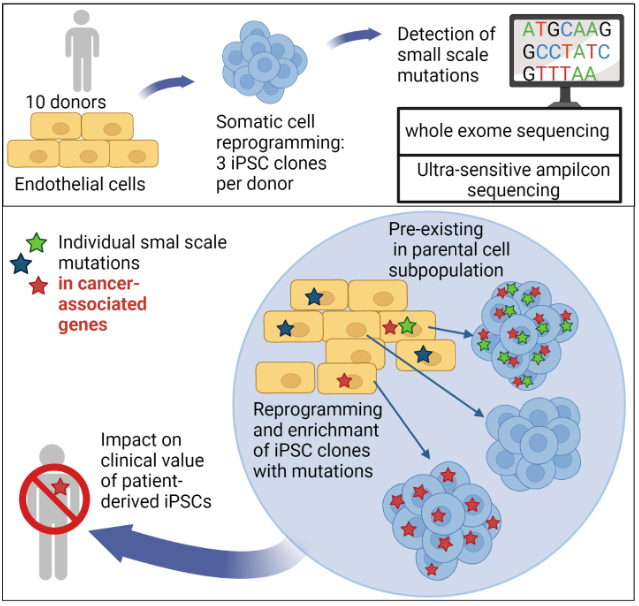
In recent years it has become clear that iPS cells may carry increased numbers of small genetic abnormalities at the nucleotide level and as well as some larger genetic abnormalities.
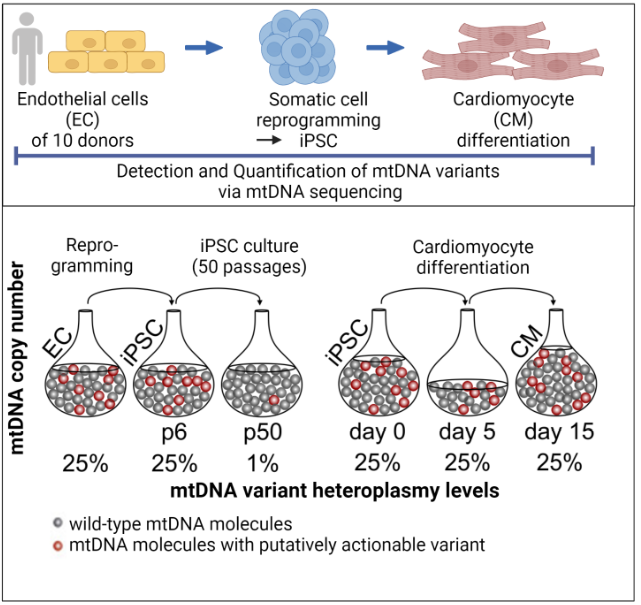
We were recently able to show that the reprogramming of somatic cells does not generate any new mutations, but that problematic mutations in the nuclear genome (Kosanke et al.,2021, Mol Ther) and in the mitochondrial genome (Kosanke et al., 2021, Stem Cell Reports) can potentially accumulate during the reprogramming and following expansion process.
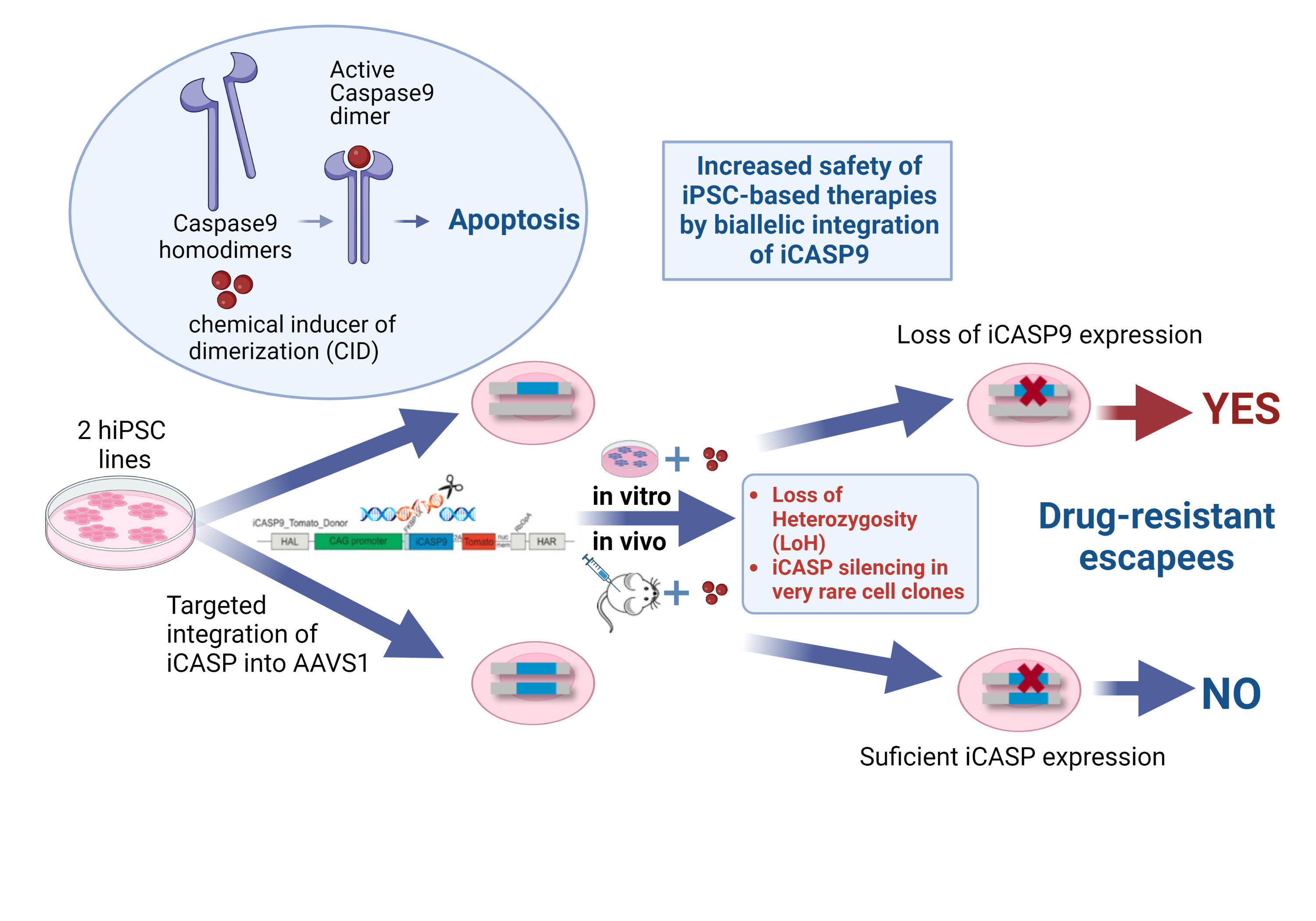
In order to address further safety aspects of iPSC-based therapies, we investigate the application of so-called suicide genes as inducible safety switches following their introduction into cells by genetic engineering.
Using this method, we were able to show that the integration of one copy of the inducible caspase 9 suicide gene (iCASP9) into the AAVS1 safe harbor locus efficiently indeed triggers cell apoptosis in vitro and in vivo, but only the integration of two copies of the suicide gene prevents the emergence of very rare apoptosis-resistant cell clones. Only in this way can the therapeutic safety level be increased through the targeted elimination of iPS cells and their derivatives after implantation (Wunderlich et al.,2022, Mol Ther Methods Clin Dev)
References:
Kosanke M, Osetek K, Haase A, Wiehlmann L, Davenport C, Schwarzer A, Adams F, Kleppa MJ, Schambach A, Merkert S, Wunderlich S, Menke S, Dorda M, Martin U. Reprogramming enriches for somatic cell clones with small-scale mutations in cancer-associated genes. Mol Ther. 2021 Aug 4;29(8):2535-2553. doi: 10.1016/j.ymthe.2021.04.007. Epub 2021 Apr 6. PMID: 33831558; PMCID: PMC8353200.
Kosanke M, Davenport C, Szepes M, Wiehlmann L, Kohrn Tim, Dorda M, Gruber J, Menge K, Sievert M, Melchert A, Gruh I, Göhring G, Martin U. iPSC culture expansion selects against putatively actionable mutations in the mitochondrial genome. Stem Cell Reports. 2021 Oct 16:2488-2502. doi: 10.1016/j.stemcr.2021.08.016
Wunderlich S, Haase A, Merkert S, Jahn K, Deest M, Frieling H, Glage S, Korte W, Martens A, Kirschning A, Zeug A, Ponimaskin E, Göhring G, Ackermann M, Lachmann N, Moritz T, Zweigerdt R, Martin U. Targeted biallelic integration of an inducible Caspase 9 suicide gene in iPSCs for safer therapies. Mol Ther Methods Clin Dev. 2022 May 31;26:84-94. doi: 10.1016/j.omtm.2022.05.011. PMID: 35795779; PMCID: PMC9234009.
6. Development of methods for in vivo correction of disease-specific mutations via "gene editing"
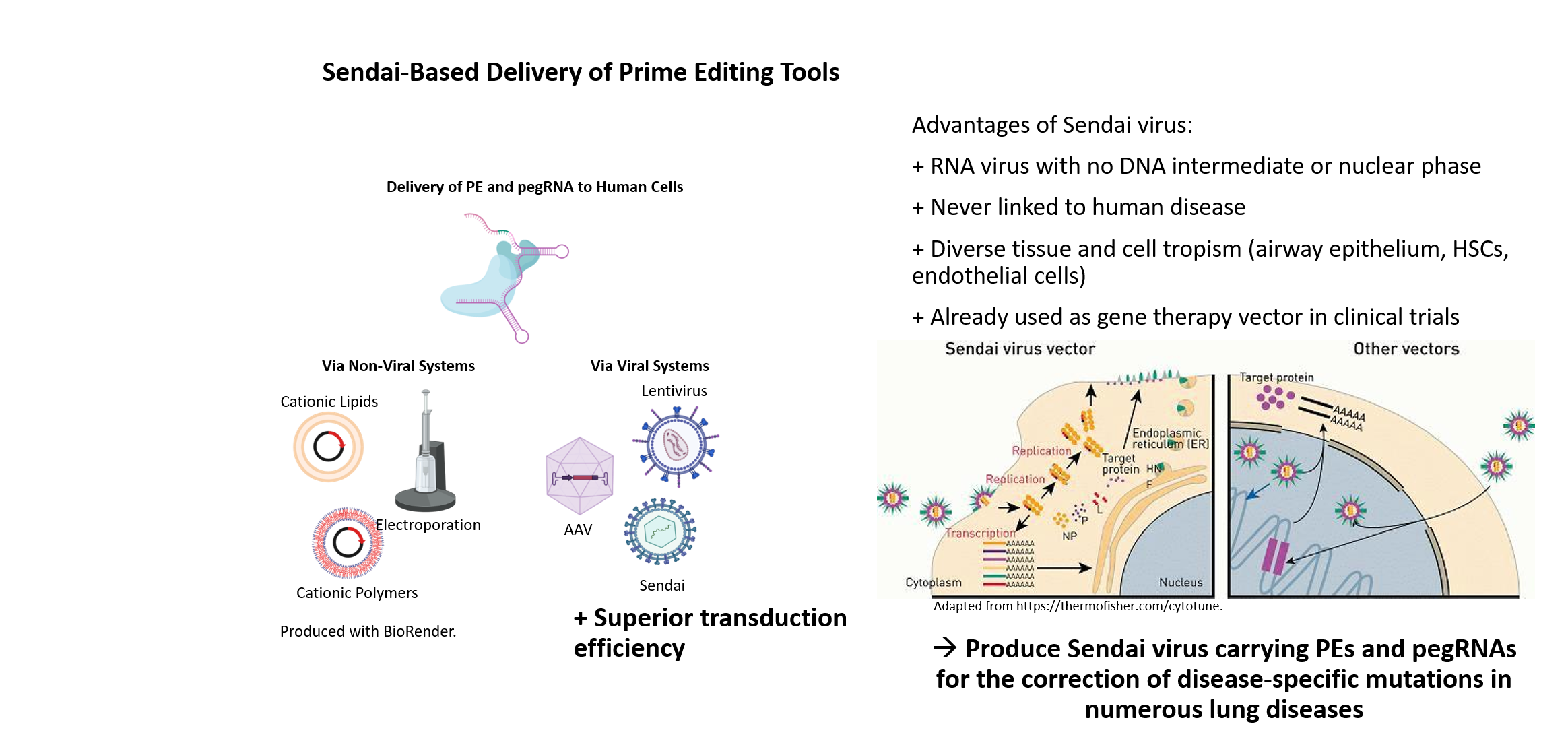
Many human genetic diseases are caused by point mutations in the genome. One approach for the correction of disease-specific mutations in targeted gene therapy is using CRISPR/Cas9-mediated gene editing, particularly the precise and versatile prime editing. For individualized treatment of patients, the development of innovative methods for efficient in vivo gene correction is of particular importance. The investigation of safe and sufficiently efficient systems to deliver the CRISPR correction machinery into patient cells is also essential for the success of gene editing in vivo. Therefore, we are establishing a novel and highly effective non-human pathogenic, non-integrating Sendai virus-based vector system for the transfer of the CRISPR/Cas9 gene editing system in vivo.
Sendai viral vectors for efficient prime editing and intravascular genetic repair of PAH disease-specific mutations
Site-directed nucleases are considered as major breakthrough in the development of regenerative therapies and biomedical research. With the advancement of ZFNs, TALENs and the CRISPR/Cas9 technology, straightforward and precise manipulation of the genome of human cells became possible. In contrast to ZFN- and TALEN-based methods, the power and attractiveness of the CRISPR/Cas9 system for biological research lies in the binding specificity of a simple Watson-Crick base pair matching between a short guide RNA and the DNA target sequence, directing the Cas9 endonuclease to cleave the DNA double strand. The specific recognition ability of CRISPR/Cas9 can also be employed for many other research applications. For instance, prime editing or base editing can be utilized to insert or correct point mutations, and a deactivated Cas9 (dCas9) can be fused to activators or repressors allowing for transcriptional regulation of distinct genes.
Although non-viral delivery systems for gene targeting in human iPSCs are quite efficient, viral delivery systems have clear advantages especially regarding in vivo targeting approaches. To exclude unwanted integration into the host genome, we have established a Sendai virus (SeV) system, an RNA virus with no DNA intermediate and no nuclear phase in its lifecycle. SeV has never been linked to human disease and readily infects many tissue and cell types including airway epithelium, hematopoietic stem cells, macrophages or endothelial cells. Besides its safety, SeV can accommodate and express foreign genes, which led to its application as gene therapy vector in clinical trials and as commercial vector for induction of pluripotency.
Our long term experience in TALEN- and CRISPR/Cas9-based genome engineering technology in combination with our induced pluripotent stem cell (iPSC) technologies will allow for the establishment of a SeV-based delivery of CRISPR/Cas9 for efficient gene editing in vivo, especially in respiratory and vascular cells. This will provide the basis for the development of new treatment options, in particular targeting lung diseases.