Methods
- Molecular and Cell Biology: mammalian cell cultures; human oocytes; primary neuronal and glial cells; D. discoideum; immunohictochemistry; transfection
- Spectroscopy: Absorption and Fluorescence Spectroscopy Techniques; Time-resolved Spectroscopy; Polarization Spectroscopy; Circular Dichroism (CD).
- Kinetics and Thermodynamics: Stopped-flow; Quench flow; Microscale Thermophoresis (MST); Calorimetry (DSC and ITC); Dynamic Light Scattering (DLS).
- Microscopy: Fluorescence microscopy, DIC, TIRFM, Confocal Microscopy, Fluorescence Life Time Imaging (FLIM), Fluorescence Resonance Energy Transfer (FRET); Fluorescence Correlation Spectroscopy (FCS), Live Cell Imaging; Single Molecule Microscopy.
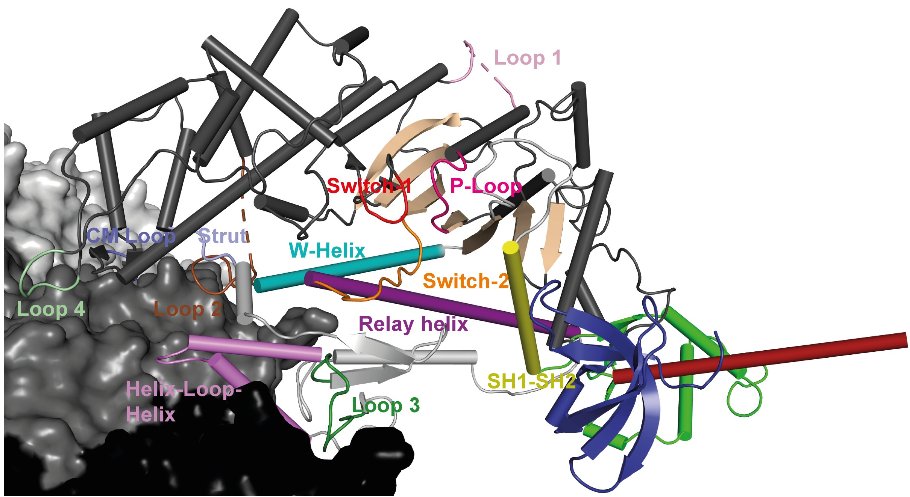
- Molecular Engineering: Molecular Modeling and Engineering of myosin-based nanomachines.
- Protein Biochemistry: Eukaryotic protein expression; native and recombinant protein production (E. coli, D. discoideum, Sf9-cells); Fluorescence Labeling techniques; gene silencing; stable gene expression; polyclonal antibody production.
- Functional Assays: Determination and characterization of the kinetic mechanisms of enzymes and proteins; ATPase measurements and kinetic equilibrium measurements, phosphate sensors, MST based studies of enzymatic reactions and enzyme kinetics; actin filament sliding and processivity assays; TIRF-based studies of actin and microtubule dynamics (polymerization, assembly, stability, depolymerization, binding partners), single molecule interactions, protein-lipid interaction studies.
- In cooperation: X-ray crystallography, Molecular Dynamics.