
Baby Lion Study
Ultra-rapid whole genome sequencing in critically ill children

05/2022 - 04/2024
Rare genetic diseases are a major cause of critical illness and mortality in children (Wright, 2018). Symptoms in young children are often acute but may be atypical or incomplete, making clinical diagnosis challenging. Ultra-rapid genome sequencing has proven to be of great clinical value in pediatric precision medicine, offering a high diagnostic rate and a short median time to diagnosis in several healthcare systems, including those in the USA (Petrikin, 2018), Canada (Elliot, 2019), and the United Kingdom (French, 2019). However, no such feasibility study has yet been conducted in Germany.
The Baby Lion study aimed to determine whether ultra-rapid whole genome sequencing (with a maximum of 5 days to a final diagnostic report) for critically ill children is feasible in routine practice in Germany.
DRKS-ID: DRKS00025163
Study cohort and inclusion criteria
130 children and their parents (trio) were enrolled in NICU/PICU in multiple centers in northern Germany. The inclusion criteria were:
- younger than 15 years
- no aetiological diagnosis
- suspected (rare) genetic disorder
Baby Lion Process
Our critically ill patients needed a reliable diagnosis in the shortest possible time. We therefore introduced an streamlined process and were able to complete the clinical report on the analyzed genome sequencing data in just Ø 4.9 days.
>> NICU/PICU: Patient selection
>> Eligibility approved by 1st interdisciplinary conference
>> informed consent, blood sampling
>> sample preparation for sequencing (~ 5:30 h)
>> rapid trio genome sequencing (~ 30:00 h)
>> bioinformatical analysis (~ 06:00 h)
>> diagnostic evaluation
>> results discussed in 2nd interdisciplinary conference
>> preliminary clinical report (after Ø 2.3 days)
>> final clinical report (after Ø 4.9 days)
Proven clinical utility of ultra-rapid genome sequencing
To evaluate the clinical utility of the entire process, the NICU/PICU staff then completed the standardized C-GUIDE™ online questionnaire (Hayeems 2023). It comprises 17 questions on indication-related outcomes, and a positive overall score indicates clinical actionability, e.g. a change in medication.
The mean C-GUIDE score for diagnostic outcomes in the Baby Lion study was 18.5 and for non-diagnostic outcomes 7.9, so overall some clinical benefit was found even for non-diagnostic outcomes. It is noteworthy that the difference between diagnostic and non-diagnostic results is highly significant.
Thus, a clinical benefit of ultra-rapid genome sequencing could be demonstrated when used for critically ill children and led to a change in management in 42% of cases.
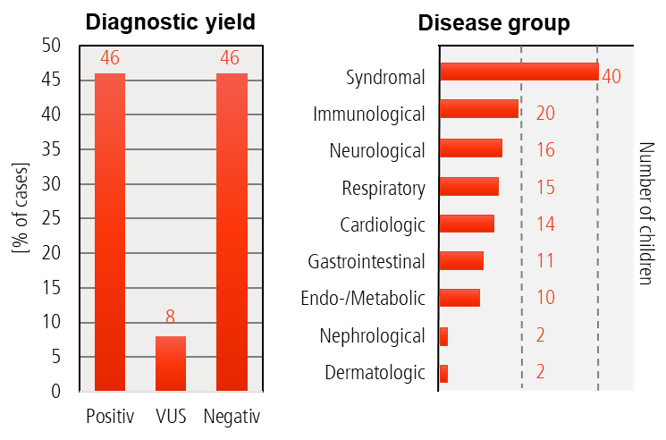
Baby Lion Results
- 130 children enrolled: ♀ 63 (48%) | ♂ 67 (52%)
- Age Ø 1.3 years
- Diagnostic yield: 46% positive | 8% variants of unknown significance (VUS) | 46% negative
- 42% of genetically diagnosed cases with change in management.
- Most children suffered from syndromic diseases, but immunologic, neuropediatric, respiratory and cardiac phenotypes were also quite common.
Conclusions
Ultra-rapid genome sequencing...
... is often the only possiblity to diagnose critically ill children in a timely manner.
... shows consistently high diagnostic yield and impact on patient management.
... should be available for every critically ill child in an interdisciplinary approach.
... is indispensable for diagnosing uncommon rare diseases.



